UltraGRO™-PURE GMP grade
Product Type:FDhPL
Attribute:Xeno-free
Grade:GMP grade
Product Name:UltraGRO™-PURE
Catalog Number:HPCHXCGL50, HPCHXCGL10, HPCHXCGL05
-
Description:
UltraGRO™-PURE cell culture supplement is a fibrinogen depleted, xeno-free supplement for replacing FBS (fetal bovine serum) to support cell expansion from research and clinical trials to commercial use application.UltraGRO™-PURE contains abundant growth factors and cytokines necessary for research or industrial cell growth and proliferation of multiple cell types (e.g. MSCs) in research-scale or industrial-scale production.
-
Quality Control Test:
1. Appearance: Yellow to Buff liquid
2. Total Protein: 4.0 - 8.0 g/dL
3. pH: 6.5 - 8.5
4. Osmolality: 275 - 355 mOsm/Kg
5. Endotoxin: ≤ 10 EU/mL
6. Mycoplasma: Not Detected
7. Sterility: No Growth
8. Performance Test: Test and record
9. Fibrinogen: < 20 μg/mL
-
Storage and Shipping Information:
UltraGRO™-PURE is most stable when stored frozen until needed. The recommended storage temperature is -20°C. Thaw frozen UltraGRO™-PURE product in 37°C water bath before use. Once UltraGRO™-PURE is thawed, it is recommended to fully use for completed medium preparation (e.g. 5%) the same day, or to divide it into single-use aliquots and store unused aliquots at -20°C.
-
Packaging Information:
500mL, 100mL, 50mL plastic bottles
-
Media Form:
Frozen Liquid
-
Raw Material:
UltraGRO™-PURE can be used as raw material in Medicines1 or Medicinal agents2 for human use: dermatology (hair growth3, preventing hair loss3,4, damaged skin5, skin care6, inflammatory system disorders7); ophthalmology (eye disease2,8); orthopedics (muscle injuries9, cartilage and bone injuries and regeneratioin10); facilitate teething11; injection filling (wadding) for medical use12,13, blood plasma14 and albuminous15.
References:
1. Randomised double-blind placebo controlled trial of topical autologous platelet lysate in venous ulcer healing. Eur J Vasc Endovasc Surg. 2000; 20(3):296-301.
2. Blood components for topical use in tissue regeneration: evaluation of corneal lesions treated with platelet lysate and considerations on repair mechanisms. Blood Transfus. 2010; 8(2): 107–112.
3. Blood components for topical use in tissue regeneration: evaluation of corneal lesions treated with platelet lysate and considerations on repair mechanisms. Blood Transfus. 2010; 8(2): 107–112.
4. Alopecia and platelet-derived therapies. Stem Cell Investig. 2017; 4: 88.
5. Analysis of Reparative Activity of Platelet Lysate: Effect on Cell Monolayer Recovery In Vitro and Skin Wound Healing In Vivo. Bull Exp Biol Med. 2016; 162(1):138-145.
6. Effect of platelet lysate on human cells involved in different phases of wound healing. PLoS One. 2013; 8(12):e84753.
7. Dual effect of platelet lysate on human articular cartilage: a maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng Part A. 2013; 19(11-12):1476-88.
8. Animal models to assess the therapeutic efficacy of human serum and serum-converted platelet lysates for dry eye syndrome: Seeing is believing. Transfus Apher Sci. 2015; 53(1):95-8.
9. Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study. Int. J. Mol. Sci. 2018, 19(1), 212.
10. Assessment of bone healing ability of calcium phosphate cements loaded with platelet lysate in rat calvarial defects. J Biomater Appl. 2016; 31(5):637-649.
11. The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars. Biomaterials. 2012; 33(20):5023-35.
12. Autologous platelet lysate local injections for the treatment of refractory lateral epicondylitis. J Orthop Surg Res. 2016; 11:17.
13. Safety and Efficacy of Autologous Intra-articular Platelet Lysates in Early and Intermediate Knee Osteoarthrosis in Humans: A Prospective Open-Label Study. Clin J Sport Med. 2015; 25(6):524-8.
14. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013; 24(3):173-82.
15. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016: 76:371-87.
-
Application:
For human ex-vivo tissue and cell culture processing applications.
-
Instructions for Use:
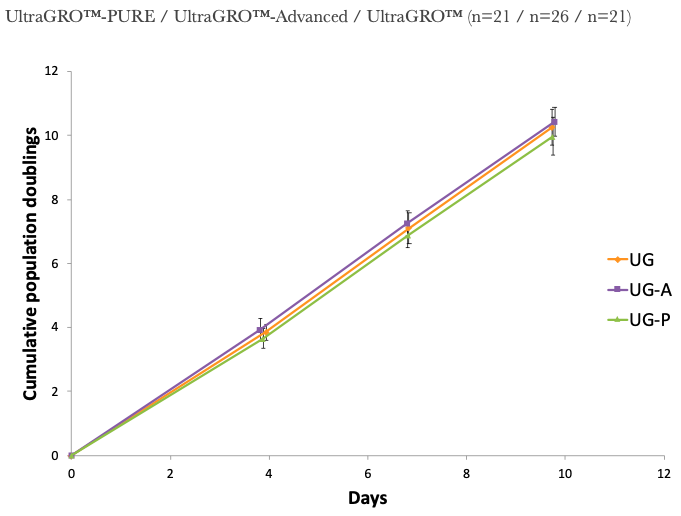
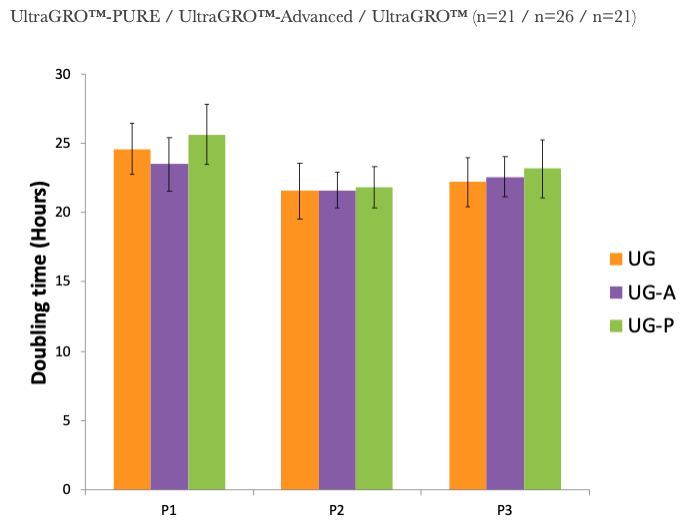
1. UltraGRO™-PURE shows optimal growth of MSC at 5% (v/v) in typical cell culture media, i.e. α-MEM, which contains 2mM L-Glutamine as final concentrate.
2. We recommend seeding MSCs at approximately 3x103~6x103 per cm2.
3. UltraGRO™-PURE has been fibrinogen-depleted and does not require the addition of heparin in the cell culture media.
-
Cell Lines:
bone marrow mesenchymal stem cells, bdipose tissue derived mesenchymal stem cells, umbilical cord derived mesenchymal stem cells, other mesenchymal stem cells
-
Important Information:
1. Insoluble particles may form in thawed UltraGRO™-PURE. Published research has shown that particles will not alter the performance of the product.
2. Insoluble particles may form in thawed UltraGRO™-PURE, it is recommended to centrifuge at 3,400 xg for 3~5 minutes.
3. Filtering the completed medium (e.g. 5%), after UltraGRO™-PURE is diluted in the basal medium, will not affect UltraGRO™-PURE supplemented cell culture performance. However, 0.22 μm filtering is NOT recommended for the 100% UltraGRO™-PURE concentrate, as this may reduce 5% UltraGRO™ -PURE cell culture performance.
4. Repeated freeze-thaw cycles should be avoided as they will cause an increase in insoluble precipitates and resulting potential decrease in UltraGRO™-PURE performance.
-
Safety information:
1. Follow the handling instructions outlined in the Material Safety Data Sheets (MSDSs). Wear appropriate protective eyewear, clothing, and gloves.
2. UltraGRO™-PURE is a cell culture supplement derived from human platelets collected from healthy donors at FDA licensed blood centers. Each donor has been tested using FDA licensed tests and found nonreactive for HBsAg, Hepatitis B core antibody (anti-HBc), HIV antibody (anti-HIV-1/2), Hepatitis C antibody (anti-HCV), HTLV-1/2 antibody (anti-HTLV-1/2), Trypanosoma cruzi antibody (anti-T. cruzi), HIV-1, HCV, HBV, WNV nucleic acid testing and Syphilis microhemagglutination test.
3. This product is manufactured, tested and released in compliance with the relevant GMP guidelines. This product is for ex-vivo use only.
-
US FDA Drug Master File:
UltraGRO™-PURE has filed a Type II Drug Master File (DMF). Please contact sales@atcbiomed.com for further information.